These NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame Questions and Answers are prepared by our highly skilled subject experts to help students while preparing for their exams.
Combustion and Flame NCERT Solutions for Class 8 Science Chapter 6
Class 8 Science Chapter 6 Combustion and Flame Textbook Exercise Questions and Answers
Page – 75
Question 1.
List conditions under which combustion can take place.
Answer:
Following are the conditions under which combustion can take place:
- The presence of fuel is necessary.
- The presence of air or oxygen.
- Heat should be supplied to raise the temperature of the fuel beyond its ignition temperature.
Question 2.
Fill in the blanks:
a. Burning of wood and coal causes …………… of air.
b. A liquid fuel, used in homes is ……………
c. Fuel must be heated to its …………… before it starts burning.
d. Fire produced by oil cannot be controlled by ……………
Answer:
(a) pollution,
(b) kerosene,
(c) ignition temperature,
(d) water
Question 3.
Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
The use of diesel and petrol as fuels in automobiles is a major cause of air pollution today. During combustion, these fuels release a large amount of unburnt carbon particles which are highly poisonous and harmful for living beings and the environment. These fine particles are dangerous pollutants causing respiratory diseases, such as asthma. Incomplete combustion of these fuels gives off carbon monoxide gas. It is a very poisonous gas.
Combustion of most fuels releases carbon dioxide in the environment. Increased emission of carbon dioxide in the air is one of the major causes for global warming. The use of diesel and petrol as fuels in automobiles is being replaced by CNG (Compressed Natural Gas), because CNG produces the harmful products in very small amounts. CNG is a cleaner fuel. It has high fuel efficiency. Hence use of CNG in automobiles has reduced pollution in our cities to a noticeable extent.
Question 4.
Compare LPG and wood as fuels.
Answer:
Wood has traditionally been used as a kitchen fuel and is still predominantly being used in rural areas. Burning of wood releases many air pollutants which can result in respiratory problems. Moreover, incomplete oxidation during burning of wood releases carbon monoxide gas which is a poisonous gas. LPG is a much better fuel because it bums without giving any smoke. It produces lesser amount of air pollutants. Complete oxidation during burning of LPG does not lead to the formation of carbon monoxide gas.
![]()
Question 5.
Give reasons:
a. Water is not used to control fires involving electrical equipment.
b. LPG is a better domestic fuel than wood.
c. Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer:
a. Pure water is a bad conductor of electricity but normal water contains many salts and hence is a good conductor of electricity. Trying to put off fires involving electrical equipment with water can result in electric shock. Due to this, water is not used to control fires involving electrical equipment.
b. LPG is a better domestic fuel than wood because of several reasons. Unlike wood, LPG bums without smoke. This makes the life of the cook more comfortable and they do not have to worry about blackening of pots and pans. Moreover, use of LPG as domestic fuel also rules out the chances of getting respiratory disorders which may happen when someone uses wood as kitchen fuel. Storage and transportation of LPG is easier as compared to that of wood,
c. The ignition temperature of paper is lower as compared to that of aluminium. When paper is wrapped around an aluminium pipe, the ignition temperature increases. That is why paper itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Question 6.
Make a labelled diagram of a candle flame.
Answer:
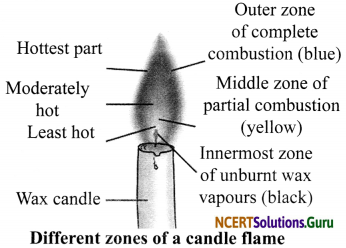
Question 7.
Name the unit in which the calorific value of a fuel is expressed.
Answer:
Kilojoule per kilogram (kJ/kg).
Question 8.
Explain how CO2 is able to control fires.
Answer:
Carbon dioxide is heavier than oxygen. Due to this, carbon dioxide forms a blanket around the burning material and makes a barrier between the burning material and oxygen. As oxygen supply is cut off, it helps in extinguishing the fire.
Question 9.
It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
Green leaves contain moisture and as a result, the ignition temperature of green leaves is much higher than that of dry leaves. Due to this, it is difficult to bum a heap of green leaves but dry leaves catch fire easily.
Question 10.
Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer:
The outermost zone of a flame is the hottest zone. Due to this, goldsmith uses the outermost zone of a flame for melting gold and silver.
![]()
Question 11.
In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Answer:
Total mass of the fuel = 4.5 kg
Heat produced by burning the given mass of fuel = 180,000 kJ.
We know that calorific value of fuel = \(\frac{\text { Heat produced in } \mathrm{kJ}}{\text { Total mass burnt }}\)
= \(\frac{180,000 \mathrm{~kJ}}{4.5 \mathrm{~kg}}\) = 40,000 kJ/kg
Hence, the calorific value of the given fuel is 40,000 kJ/kg.
Question 12.
Can the process of rusting be called combustion? Discuss.
Answer:
Although the process of rusting also involves oxidation, yet it cannot be termed as combustion. The reason for this is that combustion is defined as a process in which oxidation is accompanied by heat, and heat is not produced during rusting.
Question 13.
Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Answer:
Since Abida has kept the beaker in the luminous zone of the flame, the beaker will take more time to get heated as this zone is moderately hot. On the other hand, Ramesh has kept the beaker in the non-luminous zone of the flame so his beaker will be heated in shorter time as this is the hottest zone of the flame.
NCERT Learning Activities and Projects
Question 1.
Survey the availability of various fuels in your locality. Find out their cost per kg and prepare a tabular chart showing how many kJ of various fuels you can get for every rupee.
Hint:
Do it yourself.
Question 2.
Find out the number, type and location of fire extinguishers available in your school, nearby shops and factories. Write a brief report about the preparedness of these establishments to fight fire.
Hint:
Basically there are two types of fire extinguishers. They are carbon dioxide fire extinguishers and water fire extinguisher.
Others may include water and foam, dry chemical, wet chemical, clean agent, water mist, etc.
Carbon dioxide fire extinguisher is used when there are fires caused by oil, petrol
or by any electric appliance. Water fire extinguisher is used to fight fires caused by wood or paper.
![]()
Question 3.
Survey 100 houses in your area. Find the percentage of households using LPG, kerosene, wood and cattle dung as fuel.
Hint:
Do it yourself.
Question 4.
Talk to people who use LPG at home. Find out what precautions they take in using LPG.
Hint:
The following precautions must be taken while using LPG cylinders:
a. Before igniting the gas, we should make sure that there is no foul smell of the leaking gas. If there is a foul smell of gas, then the doors and windows should be opened at once to allow the gas to escape. The gas cylinder, rubber tubing and gas stove should then be properly checked to detect the cause of gas leakage. Preferably, a mechanic should be called to fix the defect. The gas should be used only after the defect has been rectified. Never use a leaking gas cylinder.
b. We should not use any open flames, like a kerosene stove or an electric heater, near the gas cylinder.
c. The rubber pipe connecting the gas cylinder to the gas stove should be checked regularly for any wear and tear.
d. The gas cylinder should be handled with care to avoid any damage to the cylinder valve.
e. The cylinder valve should be closed when the gas stove is not in use.
f. While lighting the gas stove, we should first open the cylinder (regulator) valve and then turn the knob of the gas stove.
Question 5.
Make a model of a fire extinguisher. Place a short candle and a slightly taller candle in a small dish filled with baking soda. Place the dish at the bottom of a large bowl. Light both the candles. Then pour vinegar into the dish of baking soda. Take care. Do not pour vinegar on the candles. Observe the foaming reaction. What happens to the candles? Why? In what order?
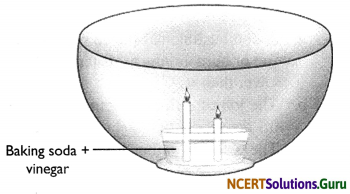
Hint:
When vinegar is combined with baking soda, the two react and produce carbon dioxide gas. The carbon dioxide gas is heavier than the surrounding air so it sinks into the bottom of the bowl. As the reaction continues, more and more carbon dioxide gas is produced which begins to fill up the bowl slowly. When the level of carbon dioxide has risen to the level of the flame, the flame will be extinguished due to lack of oxygen.
Activity 1
Objective: To show that air is necessary for combustion.
Materials Required: A lighted candle, glass chimney, a glass plate and wooden blocks.
Procedure:
- Fix a lighted candle on a table.
- Put a glass chimney over the candle and rest it on a few wooden blocks in such a way that air can enter the chimney. Observe what happens to the flame.
- Now remove the blocks and let the chimney rest on the table. Again observe the flame.
- Finally, put a glass plate over the chimney. Watch the flame again.
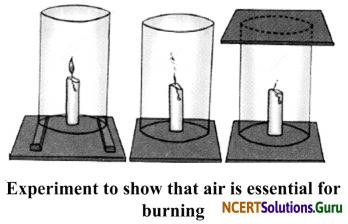
Observations:
- When we bum the candle under the chimney rested on the wooden blocks, the candle bums in the presence of air.
- When we remove the blocks, the candle flame flickers and produces smoke in the absence of air.
- When we put a glass plate on it, in the complete absence of air, the flame finally goes off.
Conclusion: In the absence of air, the candle flame goes off. Hence, air is necessary for combustion.
![]()
Activity 2
Objective: To show that a substance does not catch fire at a temperature below its ignition temperature.
Materials Required: Candle (two), sheet of paper and water.
Procedure:
- Take a sheet of paper and make two paper cups (100 mL) by folding the paper.
- Place some water into one of the cups so as to fill it up to one-third of its height.
- Hold both the cups separately over burning candle and observe.

Observations:
- When the cup is empty, it catches fire easily.
- When the cup having water in it is heated, it does not catch fire and the water becomes hot and starts boiling.
Conclusion: When we heat a paper bowl containing water, the water present in it absorbs the heat coming from the burning source and thus prevents the paper from reaching its ignition point. Hence, it does not bum.
Contents of a Modern Matchstick:
Matchsticks are in use since ages. Modem matchsticks are made up of a mixture of antimony trisulphide and potassium chlorate with some glue and starch applied on the head of the matchstick. The mbbing surface has powdered glass and some red phosphorus. On striking match stick against the rough surface, red phosphorus gets converted into white phosphorus and it reacts with potassium chlorate to ignite antimony trisulphate, due to which combustion takes place.

Types of Combustion: There are following three types of combustion:
i. Rapid Combustion: When combustion happens at a faster rate, it is called rapid combustion. Petrol and gas show rapid combustion. For example, bring a burning matchstick or gas lighter near a gas stove in the kitchen. Turn on the l knob of the gas stove. We find that the gas burns rapidly and produces heat and light.
ii. Spontaneous Combustion: When combustion starts on its own without an apparent cause, it is called spontaneous combustion. For example, many disastrous fires in coal mines result due to this kind of combustion. The heat rays coming from the sun or a lightning strike might be responsible for this kind of combustion.
iii. Explosion: When combustion reaction is so sudden that it releases a large amount of heat, light and sound, it is called explosion. Fire-crackers explode because of this type of combustion.
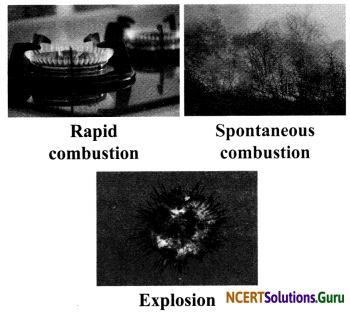
Flame: Flame is the visible and gaseous part of fire. When vapour of a substance undergoes combustion, it results in the formation of flame. Only those substances which vapourise on burning produce flame. If a substance does not get vapourised, it does not produce flame on burning.
Three Distinct Zones of a Candle Flame
- Outer Zone: This zone is blue in colour. It is the hottest part of the flame because compLete combustion takes place in this zone.
- Middle Zone: This zone is yellow or orange in colour. This zone is moderately hot because partial combustion takes place in this zone. It contains unburnt carbon particles.
- Inner Zone: This zone is dark in colour. This is the least hot part of the flame because no combustion takes place in this zone. Here, we can find the unburnt wax vapours of a candle.
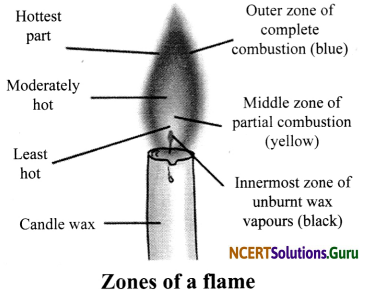
![]()
Activity 3
Objective: To show the presence of unburnt carbon particles in the middle zone of the candle flame.
Materials Required: A wax candle, matchbox, glass slide and a pair of tongs.
Procedure:
- Light the candle and allow the flame to become steady.
- Introduce a clean glass slide into the luminous region of the flame holding it with a pair of tongs.
- Remove the glass slide after 3-4 minutes.
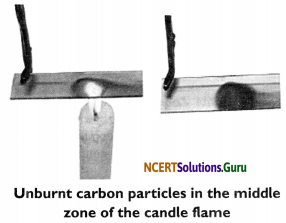
Observation: A circular grey ring forms on the glass.
Conclusion: The circular dark ring is formed on the slide by the deposition of unburnt carbon particles present in the luminous (middle) zone of the candle flame.
Fuel: The substance that undergoes combustion is called a fuel. The fuel can be in the form of solid, liquid or gas.
- Liquid Fuels: Petroleum, crude oil, diesel, kerosene oil, etc.
- Solid Fuels: Firewood, charcoal, coal, dung cake, tallow (animal fat), straw and other agricultural wastes, paraffin wax, camphor, etc.
- Gaseous Fuels: Most commonly used gas, i.e. LPG (Liquid Petroleum Gas) which we use as cooking gas at home and CNG (Compressed Natural Gas).
Characteristics of a Good Fuel:
- It should be easy to store and transport.
- It should bum smoothly without emitting harmful gases.
- It should not produce any smoke.
- It should have moderate ignition temperature.
- It should have high calorific value.
There is probably no fuel that could be considered as an ideal fuel.
Calorific Value: Calorific value gives the measure of fuel efficiency. The amount of heat energy produced on combustion of 1 kg of a fuel is called the calorific value of that fuel. It is expressed as kilo Joule per kg (kJ/kg).
| Fuel | Calorific Value (kJ/kg) |
| Cow dung cake | 6000-8000 |
| Wood | 17000-22000 |
| Coal | 25000-33000 |
| Petrol | 45000 |
| Kerosene | 45000 |
| Diesel | 45000 |
| Methane | 50000 |
| CNG | 50000 |
| LPG | 55000 |
| Biogas | 35000-40000 |
| Hydrogen | 150000 |
Fire Extinguishers: A substance which disrupts the contact between air and fire so that the fire goes off is called a fire extinguisher. Some common fire extinguishers are as follows:
i. Water: Water is the most commonly used fire extinguisher. It helps in bringing down the ignition temperature of the fuel. When water is poured over a burning material, steam is formed due to heat. The layer of steam cuts off air supply. Thus, water helps in putting off fire.

Drawbacks of Water: Water cannot be used for fires due to oil because oil is lighter than water. When water is poured over fire, oil comes on top and continues to bum. Also, water should not be used for electric fires. We know that water is a good conductor of electricity because it contains many salts. This increases the risk of electric shock for the firefighters.
ii. Blanket: If fire is at a small scale, a blanket can be very useful in controlling the fire. When the burning object is covered with blanket, the oxygen supply is cut off from the fuel. This helps in putting off fire.

iii. Carbon Dioxide: This is the best fire extinguisher. Carbon dioxide creates a blanket over fire and cuts off air supply because it is heavier than air. Carbon dioxide expands quickly and brings down the temperature of the fuel. This helps in putting off fire.

For extinguishing fire, carbon dioxide can be supplied in any of the following ways:
- Carbon dioxide is stored in pressurised cylinders and can be released through a nozzle.
- In soda-acid fire extinguishers, carbon dioxide is evolved because of reaction between soda and acid.
- Baking soda (sodium bicarbonate) or potassium bicarbonate powder is sprayed on the burning substance. The powder releases carbon dioxide because of heat.
Harms of Burning Fossil Fuels:
- Burning of carbon fuels (wood, coal and petroleum) results in the release of unburnt carbon particles in air. These particles cause respiratory disease, like asthma.
- Incomplete combustion of carbon fuels releases carbon monoxide. It is a poisonous gas, even in low concentrations. Hence, coal should not be burnt in a closed room.
- Burning of most fuels results in release of carbon dioxide in the atmosphere. Higher level of carbon dioxide in the atmosphere causes global warming.
- Burning of coal, and diesel results in the release of sulphur dioxide in the air. Burning of petrol produces oxides of nitrogen. Oxides of sulphur and nitrogen mix with rainwater to cause acid rain. Acid rain is harmful for living beings, buildings, crops and for monuments.
Class 8 Science Chapter 6 Combustion and Flame Additional Important Questions and Answers
Very Short Answer Type Questions
Question 1.
Define combustion.
Answer:
Combustion is a chemical process in which a substance reacts with oxygen to give off heat.
Question 2.
Give examples of some fuels used in our day to day life.
Answer:
Cow dung, coal, wood, petrol, charcoal, compressed natural gas (CNG), etc.
Question 3.
What are the products of combustion?
Answer:
Carbon dioxide, water vapour, heat and light.
Question 4.
What is a combustible substance?
Answer:
A substance which can undergo combustion is called a combustible substance, e.g., wood, paper, kerosene, etc.
![]()
Question 5.
What is a non-combustible substance?
Answer:
A substance which cannot undergo combustion is called a non-combustible substance, e.g., stone, soil, etc.
Question 6.
What is an inflammable substance?
Answer:
A substance which has very low ignition temperature and can easily catch fire is called an inflammable substance, e.g. petrol, alcohol.
Question 7.
What is a flame?
Answer:
Flame is the visible and gaseous part of the fire formed by the burning of vapours of fuel.
Question 8.
What is the colour of LPG flame?
Answer:
The colour of LPG flame is blue.
Question 9.
What is a fire extinguisher?
Answer:
The substance which is used for putting off fire is called fire extinguisher.
Question 10.
Give an example of rapid combustion.
Answer:
When we bring a burning matchstick or gas lighter near a gas stove and turn on the knob of stove, the gas bums rapidly and produces heat and tight. This is an example of rapid combustion.
![]()
Question 11.
What is the principle to extinguish fire?
Answer:
Fire can be controlled by removing one or more of the essential requirements-air, fuel or heat.
Question 12.
Name two different types of fire extinguishers.
Answer:
- Soda-acid fire extinguisher (contains baking soda and acid),
- Fire extinguisher cylinders having CO2 stored at high pressure.
Question 13.
Give examples of the substances which give off flame on burning.
Answer:
Kerosene and molten wax.
Question 14.
Give two properties of an ideal fuel.
Answer:
High calorific value and low cost.
Question 15.
Which chemicals give rise to acid rain?
Answer:
Sulphur dioxide and nitrogen oxides.
Short Answer Type Questions
Question 1.
Differentiate between rapid and spontaneous combustion.
Answer:
|
Rapid combustion |
Spontaneous combustion |
| a. It needs to be initiated once. | a. It takes place by itself. |
| b. External heat is required to start it once. | b. No external heat is required to start it. |
| c. Large amount of heat and light is evolved in a short time. | c. Small amount of heat and light is evolved. |
| d. Example: Burning of domestic cooking gas in a gas burner. | d. Burning of white phosphorus on its own when exposed to air for some time. |
![]()
Question 2.
Why does kerosene oil catch fire faster than wood?
Answer:
This is because the specific heat capacity of the wood is more than that of the kerosene oil. Hence, the wood takes time to bum but burns for a longer period than the kerosene oil.
Question 3.
How many types of combustion are there? Explain them.
Answer:
There are three types of combustion:
- Rapid Combustion: When combustion happens at a faster rate, it is called rapid combustion. Petrol and gas show rapid combustion.
- Spontaneous Combustion: When combustion starts on its own, without any apparent cause, it is called spontaneous combustion. For example, coal dust in coal mines can start burning on its own. Forest fires are spontaneous in most of the cases.
- Explosion: When combustion reaction is so sudden that it releases a large amount of heat, light and sound, it is called an explosion. Firecrackers explode because of this type of combustion.
Question 4.
Explain the different zones of a candle flame with the help of a labelled diagram.
Answer:
A flame has three zones:
- Outermost zone is blue in colour and is the hottest part of the flame. This is also the zone where complete combustion takes place.
- Middle zone is yellow in colour and is moderately hot. In this zone, partial combustion takes place.
- Innermost zone is black in colour and least hot. Here, we can find the unburnt wax vapours of a candle.
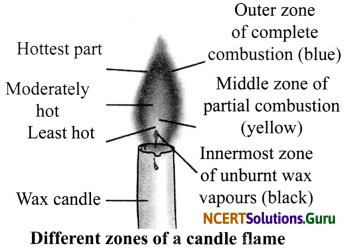
Question 5.
State the characteristics of a good fuel.
Answer:
The characteristics of a good fuel are:
- It is readily available.
- It is cheap.
- It bums easily in air.
- It bums at a moderate rate.
- It produces a large amount of heat.
- It does not leave behind any undesirable substance.
- It is easy to store and transport.
![]()
Question 6.
List the different methods by which a fire can be extinguished.
Answer:
A fire can be extinguished by one or more of the following methods:
- Removing the combustible substance.
- Cooling the substance to bring its temperature below its ignition temperature using water.
- Cutting off the supply of air or using fire extinguishers.
Question 7.
What is calorific value of a fuel? Why is hydrogen not used as a fuel though it has the highest calorific value?
Answer:
Calorific value: The amount of heat energy produced on complete combustion of one kilogram of a fuel is called the calorific value of that fuel. Hydrogen is not used as a fuel because it has very low ignition temperature and is highly explosive in nature.
Question 8.
How are fuels classified on the basis of their physical state? Give two examples of each.
Answer:
Fuels can be classified on the basis of their physical state as follows:
- Solid fuels: Some solid fuels are wood, coal, cattle-dung cakes, etc.
- Liquid fuels: Some of the liquid fuels are kerosene, LPG, petrol, diesel, etc.
- Gaseous fuels: Some of the gaseous fuels are CNG, coal gas, water gas, producer gas, biogas, etc.
Question 9.
Why are we advised never to sleep in a closed room with burning coal fire in it?
Answer:
In the presence of less amount of oxygen, the carbon monoxide gas is produced by incomplete combustion of coal. It is a poisonous gas and can kill the person sleeping in a closed room. Hence, we are advised not to sleep in a closed room with burning coal fire in it.
Question 10.
Why is the colour of outer zone of a candle flame blue while middle zone is yellow in colour?
Answer:
Outermost Zone: It is blue coloured because complete combustion takes place in this part due to sufficient amount of oxygen. It is the hottest part of the flame.
Middle Zone: The colour of the middle zone is yellow because incomplete combustion takes place in this part due to lack of oxygen. It is comparatively less hot than the outer part of the flame.
Question 11.
What do you mean by global warming? Write its effects.
Answer:
The increase in temperature of the atmosphere of the earth is called global warming. This causes melting of polar ice caps which leads to a rise in the sea level, causing floods in the coastal areas. Low lying coastal areas may even be permanently submerged underwater. Global warming is the result of increase of carbon dioxide concentration in the atmosphere, mostly due to combustion of fuels.
Question 12.
What can we do to cut off the air supply of a burning substance?
Answer:
To put off the air supply of a burning substance, we can cover the surface of the burning substance with sand, foam or a thick cloth like a blanket. Fire extinguishers form a layer of foam or carbon dioxide on the surface of the burning substance.
![]()
Question 13.
Why does a matchstick start burning when it is rubbed on the side of the matchbox?
Answer:
The head of the matchstick is made from antimony trisulphide and potassium chlorate. The rubbing surface has powdered glass and a little red phosphorus. When the matchstick is struck against the rubbing surface, some of the red phosphorus is converted into white phosphorus. This immediately reacts with potassium chlorate in the matchstick head to produce enough heat to ignite antimony trisulphide and starts the combustion of matchstick.
Question 14.
Why should we not use water to control oil fires? What should be done to control fire due to oils?
Answer:
Water should never be used for extinguishing oil fires. This is because oil is lighter than water. Oil forms a thin layer over the surface of water and flows with the water, thereby spreading the fire to larger areas. In such a case, sand or soil should be used. They cut off the supply of air and the fire is put off.
Question 15.
How does fire brigade work to extinguish fire?
Answer:
Firemen throw water with pressure on the fire. Water helps in cooling down of the combustible material so that its temperature is brought below its ignition temperature and the fire is controlled. Apart from this, combustible material is surrounded by water vapour which helps in cutting off the air supply and finally the fire is extinguished.
Long Answer Type Questions
Question 1.
Describe the working of soda-acid fire extinguisher.
Answer:
Soda-acid fire extinguisher: This fire extinguisher is a metallic cylinder which contains sodium bicarbonate (NaHCO3) solution. At the bottom of the cylinder, the concentrated sulphuric acid (H2SO4) is placed in a thin sealed glass tube. Fixed wire gauze surrounds this tube.
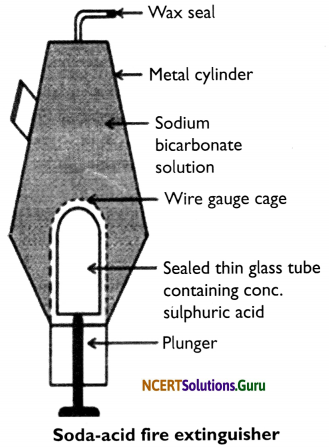
Below the tube, a plunger is placed with its sharp end touching the thin glass tube. On the top of the cylinder, there is a nozzle which is sealed with wax. When the plunger hits against the floor, its sharp end breaks the thin glass tube and the acid inside it reacts with sodium bicarbonate to produce carbon dioxide. CO2 forces the wax seal open and rushes out of the nozzle to put out the fire in the direction where the nozzle is pointed.
Question 2.
Discuss the impact of burning fuels on air quality.
Answer:
The main impacts due to burning of fuels are as follows:
a. Burning of carbon-containing fuels releases ash and fine particles of unburnt carbon in the air. These fine particles, called SPM (suspended particulate matter) are dangerous pollutants.
b. Combustion of fuels adds carbon dioxide to the environment. A percentage increase in carbon dioxide in the air leads to the greenhouse effect which can cause global warming. This can result in melting of the polar ice caps and rise in sea levels, leading to floods in low-lying areas.
c. Carbon monoxide produced when fuels containing carbon bum in insufficient supply of air, is a very poisonous gas and can cause death if breathed in.
d. Coal contains sulphur, which produces sulphur dioxide on burning. Besides being a poisonous gas, it dissolves in rain to form sulphuric acid. This gives rise to acid rain, which is very harmful for soil, crops, buildings, etc.
e. The oxides of nitrogen given off from the exhausts of engines of vehicles are poisonous gases. Many vehicles now use catalytic converters to convert these oxides to harmless gases.
f. Lead compounds are released in exhausts of vehicles. They are poisonous. Increasing use of unleaded petrol is expected to reduce lead pollution.
![]()
Question 3.
Name the fire extinguisher used for fires involving electrical equipment and inflammable materials. What are its advantages as a fire extinguisher?
Answer:
The most commonly used fire extinguisher is water. It works when wood and paper are on fire. But if electrical equipment is on fire, water may conduct electricity and may harm those trying to douse the fire. Also, water is not suitable for fires involving oil and petrol, since water is heavier than oil, so it sinks below the oil and oil keeps burning on the top. Hence, if electrical equipment and inflammable materials are on fire, carbon dioxide is the best extinguisher. Since it is heavier than oxygen, it covers the burning material so that the contact between fuel and oxygen is cut off and the fire is controlled. It also brings down the temperature of the fuel. For this purpose, carbon dioxide is stored at high pressure, as a liquid, in cylinders.
Question 4.
Describe the history of matchstick.
Answer:
The history of matchstick is very old. More than 5000 years ago, small pieces of pinewood dipped in sulphur were used as matches in ancient Egypt. The modem safety match was developed only 200 years ago. A mixture of antimony trisulphide, potassium chlorate and white phosphorus with some glue and starch was applied on the head of match made of wood. When struck against a rough surface, white phosphorus got ignited due to heat of friction. This started the combustion of matchstick. However, white phosphorus proved to be dangerous to health, both for the workers involved in manufacturing and the users. These days red phosphorus is used in place of white phosphorus.
Picture Based Questions
Question 1.
Observe the given figure and answer the following questions.

a. Name the article shown above.
b. Name two different varieties in which this article is available.
c. How does it work?
Answer:
a. Fire extinguisher.
b. Carbon dioxide and soda-acid type fire extinguisher.
c. It works by cutting off air supply to the burning fuel.
![]()
Question 2.
a. Draw a diagram to show the flame of:
(i) Candle (ii) Kerosene lamp and (iii) Bunsen burner.
b. Write the colour of these flames.
Answer:
a.
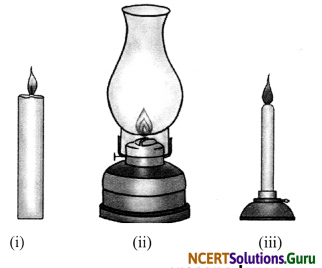
Flame of (a) candle (b) kerosene lamp, and (c) Bunsen burner
b. The colour of the flame of kerosene lamp and candle is yellow. The colour of the flame of Bunsen burner is blue.