Students may want to download NCERT MCQ Questions for Class 12 Chemistry Chapter 3 Electrochemistry with Answers Pdf free download. This Electrochemistry Class 12 MCQs Questions with Answers can help understand the concepts score better in your Class 12 Chemistry Exam, so make sure you practice these! Check out chapter-wise MCQ Questions for Class 12 Chemistry with Answers.
Electrochemistry Class 12 MCQs Questions with Answers
Solve this Electrochemistry Multiple Choice Questions of Class 12 Chemistry Chapter 3 MCQ, so as not to miss out on any concept from being clear about what they mean!
Question 1.
If the conductivity and conductance of a solution is same then its cell constant is equal to:
(a) 1
(b) 0
(c) 10
(d) 1000
Answer
Answer: (a) 1
Question 2.
The units of conductivity are:
(a) ohm-1
(b) ohm-1 cm-1
(c) ohm-2 cm² equiv-1
(d) ohm-1 cm²
Answer
Answer: (b) ohm-1 cm-1
Question 3.
The resistance of 0.1 N solution of acetic acid is 250 ohm, when measured in a cell of cell constant 1.15 cm-1. The equivalent conductance (in ohm-1 cm² equivalent-1) of 0.1 N acetic acid is
(a) 18.4
(b) 0.023
(c) 46
(d) 9.2
Answer
Answer: (c) 46
Question 4.
In infinite dilution of aqueous solution of BaCl2, molar conductivity of Ba2+ and Cl– ions are = 127.32 S cm²/mol and 76.34 S cm2/mol respectively. What is A°m for BaCI2 at same dilution?
(a) 280 S cm² mol-1
(b) 330.98 S cm² mol-1
(c) 90.98 S cm² mol-1
(d) 203.6 S cm² mol-1
Answer
Answer: (a) 280 S cm² mol-1
Question 5.
The specific conductance of 0.1 M NaCl solution is 1.06 × 10-2 ohm-1 cm-1. Its molar conductance in ohm-1 cm² mol-1 is
(a) 1.06 × 10²
(b)1.06 × 10³
(c) 1.06 × 104
(d) 53
Answer
Answer: (a) 1.06 × 10²
Question 6.
The limiting molar conductivities A° for NaCl, KBr and KCl are 126, 152 and 150 S cm² mol-1 respectively. The A° for NaBr is
(a) 278 S cm² mol-1
(b) 976 S cm² mol-1
(c) 128 S cm² mol-1
(d) 302 S cm² mol-1
Answer
Answer: (c) 128 S cm² mol-1
Question 7.
λ(CICH2COONa) = 224 ohm-1 cm² gm eq-1, λ(NaCl) = 38.2 ohm-1 cm² gm eq-1. λ(HCl) = 203 ohm-1 cm² gm eq-1, what is the value of λ(CICH2COOH)?
(a) 288.5 ohm-1 cm² gm eq-1
(b) 289.5 ohm-1 cm² gm eq-1
(c) 388.8 ohm-1 cm² gm eq-1
(d) 59.5 ohm-1 cm² gm eq-1
Answer
Answer: (c) 388.8 ohm-1 cm² gm eq-1
Question 8.
The limiting molar conductivities of HCl, CH3COONa and NaCl are respectively 425, 90 and 125 mho cm² mol-1 at 25 °C. The molar conductivity of 0.1 M CH3COOH solution is 7.8 mho cm² mol-1 at the same temperature. The degree of dissociation of 0.1 M acetic acid solution at the same temperature is
(a) 0.10
(b) 0.02
(c) 0.15
(d) 0.03
Answer
Answer: (b) 0.02
Question 9.
The values of limiting ionic conductance of H and HCOO– ions are respectively 347 and 53 S cm² mol-1 at 298 K. If the molar conductance of 0.025 M methanoic acid at 298 K is 40 S cm² mol-1, the dissociation constant of methanoic acid at 298 K is
(a) 1 × 10-5
(b) 2 × 10-5
(c) 1.5 × 10-4
(d) 2.5 × 10-4
Answer
Answer: (d) 2.5 × 10-4
Question 10.
The ionisation constant of a weak electrolyte is 2.5 × 10-5 and molar conductance of its 0.01 M solution is 19.6 S cm² mol-1. The molar conductance at infinite dilution (S cm² mol-1) is
(a) 402
(b) 392
(c) 306
(d) 39.2
Answer
Answer: (b) 392
Question 11.
Which of the following statements is incorrect about electrochemical cell?
(a) Electrons are released at anode.
(b) Chemical energy is converted into electrical energy.
(c) Salt bridge maintains the electrical neutrality of the electrolytes.
(d) Cell can work indefinitely.
Answer
Answer: (d) Cell can work indefinitely.
Question 12.
Point out the correct statement in a cell of zinc and copper:
(a) Zinc acts as cathode and copper as anode.
(b) Zinc acts as anode and copper as cathode.
(c) The standard reduction potential of zinc is more than that of copper.
(d) The flow of electrons is from copper to zinc.
Answer
Answer: (b) Zinc acts as anode and copper as cathode.
Question 13.
The standard electrode potentials for Pb2+/Pb and Zn2+/Zn are – 0.126 V and – 0.763 V respectively. The e.m.f. of the cell Zn | Zn2+ (0.1 M) | | Pb2+ (0.1 M) | Pb is:
(a) 0.637 V
(b) < 0.637 V
(c) > 0.637 V
(d) 0.889 V.
Answer
Answer: (a) 0.637 V
Question 14.
A zinc electrode is placed in 0.1 M solution of ZnSO4 at 25°C. Assuming that the salt is dissociated to an extent of 20% at this dilution, the potential of this electrode is (E° = -0.76V)
(a) – 0.81 V
(b) – 0.79 V
(c) 0.81 V
(d) 0.79 V
Answer
Answer: (a) – 0.81 V
Question 15.
For the electrode reaction Mn+(aq) + ne– → M(s) Nernst equation is
(a) E = E° + \(\frac { RT }{nF}\) ln \(\frac { [m] }{[m^{n+}]}\)
(b) E° = E° + RT ln [Mn+]
(c) E = E° + \(\frac { RT }{nF}\) ln [Mn+]
(d) \(\frac { E }{E°}\) = \(\frac { RT }{nF}\) ln [Mn+]
Answer
Answer: (c) E = E° + \(\frac { RT }{nF}\) ln [Mn+]
Question 16.
E°cell apd ΔG° are related as:
(a) ΔG° = nF E°cell
(b) ΔG = -nF E°cell
(c) ΔG° = -nF E°cell
(d) ΔG° = nF E°cell = 0.
Answer
Answer: (c) ΔG° = -nF E°cell
Question 17.
Which of the following will decrease the voltage of the cell?
Sn(s) + 2Ag+ (aq) → Sn2+ (aq) + 2 Ag(s)
(a) increase in the size of silver rod
(b) increase in the concentration of Sn2+ ions
(c) increase in the concentration of Ag+ ions
(d) None of the above.
Answer
Answer: (b) increase in the concentration of Sn2+ ions
Question 18.
In the diagram given below, the value of x is
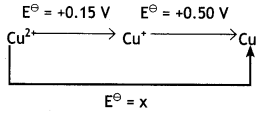
(a) 0.35 V
(b) 0.65 V
(c) 0.325 V
(d) -0.65 V
Answer
Answer: (c) 0.325 V
Question 19.
By how much will the potential of a zinc electrode change if the solution of ZnSO4 in which it is immersed is diluted to 10 times at 298 K?
(a) Decreases by 30 mV
(b) Increases by 30 mV
(c) Increases by 60 mV
(d) Decreases by 60 mV
Answer
Answer: (b) Increases by 30 mV
Question 20.
The e.m.f. of the cell:
Cu(s) | Cu2+ (1M) | | Ag+ (1M) | Ag is 0.46 V. The standard reduction potential of Ag+|Ag is 0.80 V. The standard reduction potential of Cu2+ |Cu is
(a) -0.34 V
(b) 1.26 V
(c) -1.26 V
(d) 0.34 V
Answer
Answer: (d) 0.34 V
Question 21.
Saturated solution of KNO3 is used to make salt bridge because
(a) velocity of K+ is greater than that of NO\(_3^-\)
(b) velocity of NO\(_3^-\) is greater than that of K+
(c) velocity of both K+ and NO\(_3^-\) are nearly the same
(d) KNO3 is highly soluble in water.
Answer
Answer: (c) velocity of both K+ and NO\(_3^-\) are nearly the same
Question 22.
Aluminium displaces hydrogen from acids, but copper does not. A galvanic cell prepared by combining Cu/Cu2+ and Al/Al3+ has an emf of 2.0 V at 298 K. If the potential of copper electrode is + 0.34 V, that of aluminium electrode is
(a) -2.3 V
(b) +2.34 V
(c) -1.66 V
(d) 1.66 V
Answer
Answer: (c) -1.66 V
Question 23.
Standard electrode potentials are:
Fe2+/Fe (E°= -0.44 V), Fe3+/Fe2+ (E° = 0.77 V)
Fe2+, Fe3+ and Fe blocks are kept together, then
(a) Fe3+ increases
(b) Fe3+ decreases
(c) Fe2+ |Fe3+ remains unchanged
(d) Fe2+ decreases
Answer
Answer: (b) Fe3+ decreases
Question 24.
The standard reduction potentials for two reactions are given below:
AgCl(s) + e– → Ag(s) + Cl–(aq) E° = 0.22 V
Ag+(aq) + e– → Ag(s) E ° = 0.80 V
The solubility product of AgCl under standard conditions of temperature (298 K) is given by
(a) 1.6 × 10-5
(b) 1.5 × 10-8
(c) 3.2 × 10-10
(d) 1.5 × 10-10
Answer
Answer: (d) 1.5 × 10-10
Question 25.
The e.m.f. of the following Daniell cell at 298 K is E1
In | ZnSO4 (0.01 M) | | CuSO4 (1.0) | Cu
When concentration of ZnSO4 is 1.0 M and that of CuSO4 is 0.01 M, the e.m.f. changed to E2. What is the relationship between E1 and E2?
(a) E1 > E2
(b) E1 < E2
(c) E1 = E2
(d) E2 = 0 ≠ E1
Answer
Answer: (a) E1 > E2
Question 26.
E°Cu2+/cu = 0.34 V and E°Zn2+/Zn = -0.76 V. A Daniell cell contains 0.1 M ZnSO4 solution and 0.01 M CuSO4 solution at its electrodes. The EMF of the cell is
(a) 1.10 V
(b) 1.04 V
(c) 1.16 V
(d) 1.07 V
Answer
Answer: (d) 1.07 V
Question 27.
Consider the following EΘ values E(Fe3+/Fe2+) = + 0.77 V, E(Sn2+/Sn) = -0.14 V Under standard conditions, the potential for the reaction:
Sn(s)+2Fe3+(oq) → 2Fe2+(aq) + Sn2+(aq)is
(a) 0.91 V
(b) 1.40 V
(c) 1.68 V
(d) 0.63 V
Answer
Answer: (a) 0.91 V
Question 28.
If E°(Fe2+/Fe) = -0.441 V and E°(Fe3+/Fe2+) = 0.771V, the standard E.M.F. of the reaction:
Fe + 2Fe3+ → 3Fe2+ will be
(a) 1.653 V
(b) 1.212 V
(c) 0.111 V
(d) 0.330 V
Answer
Answer: (b) 1.212 V
Question 29.
Number of coulombs required to deposit 90 g of Al when the electrode reaction Al3+ + 3e– → Al is:
(a) 9.65 × 104
(b) 8.685 × 105
(c) 9.65 × 105
(d) 6.955 × 104
Answer
Answer: (c) 9.65 × 105
Question 30.
10800 C of electricity through the electrolyte deposited 2.977 g of metal with atomic mass 106.4 a.m.u. The valency of metal cation is:
(a) 4
(b) 3
(c) 2
(d) 1
Answer
Answer: (a) 4
Question 31.
The charge on 1 gram mole ion of N3- is
(a) 6.00 × 105 C
(b) 2.89 × 105 C
(c) 3.98 × 105 C
(d) 4.89 × 105 C
Answer
Answer: (b) 2.89 × 105 C
Question 32.
A current of 3A was passed through a solution of AuCl\(_{ 4 }^{-}\) ions using gold electrodes and it caused deposition of 1.234 g of Au (Atomic mass of Au = 197). The time for which the current was passed is
(a) 20 min 8s
(b) 30 min 12s
(c) 10 min 4s
(d) 10 min 40s
Answer
Answer: (c) 10 min 4s
Question 33.
An electric current is passed through silver voltmeter connected to a water voltmeter. The cathode of silver voltmeter weighed 0.108 g more at the end of electrolysis. The volume of O2 at STP evolved is
(a) 5.6 cm³
(b) 550 cm³
(c) 22.4 cm³
(d) 11.2 cm³
Answer
Answer: (a) 5.6 cm³
Question 34.
Same amount of electric current is passed through solution of AgNO3 and HCl. If 1.08 g of silver is obtained in the first case, the amount of hydrogen liberated at S.T.P. in the second case is:
(a) 112 cm³
(b) 22400 cm³
(c) 224 cm³
(d) 1.008 g
Answer
Answer: (a) 112 cm³
Question 35.
4.5 g of aluminium (at. mass = 27. a.m.u) is deposited at cathode from Al3+ solution by certain quantity of electric charge. The volume of hydrogen produced at STP from H+ ions in solution by the same quantity of electric charge will be
(a) 44.8 L
(b) 22.4 L
(c) 11.2 L
(d) 5.6 L
Answer
Answer: (d) 5.6 L
Question 36.
The quantity of electricity needed to separately electrolyse 1 M solution of ZnSO4, AICl3 and AgNO3 completely is in the ratio of
(a) 2 : 3 : 1
(b) 2 : 1 : 1
(c) 2 : 1 : 3
(d) 2 : 2 : 1
Answer
Answer: (a) 2 : 3 : 1
Question 37.
When lead storage battery discharges
(a) SO2 is evolved
(b) PbSO4 is consumed
(c) Lead is formed
(d) H2SO4 is consumed.
Answer
Answer: (d) H2SO4 is consumed.
Question 38.
In a Leclanche dry cell, anode is:
(a) Graphite rod
(b) FeO and Fe(OH)2
(c) Zinc container
(d) MnO2 + C
Answer
Answer: (c) Zinc container
Question 39.
Rust is a mixture of
(a) FeO and Fe(OH)3
(b) FeO and Fe(OH)2
(c) Fe2O3 and Fe(OH)3
(d) Fe3O4 and Fe(OH)3.
Answer
Answer: (c) Fe2O3 and Fe(OH)3
Question 40.
Which of the following will be formed when lead storage battery is charged?
(a) Sulphuric add is consumed
(b) lead is consumed
(c) sulphuric acid is formed
(d) lead sulphate is formed.
Answer
Answer: (c) sulphuric acid is formed
Question 41.
For a H2 – O2 fuel cell, the theoretical voltage has been found to be 1.23V and ΔH to be -285 kJ mol-1. The efficiency of the fuel cell is
(a) 76%
(b) 83%
(c) 89%
(d) 72%
Answer
Answer: (b) 83%
Question 42.
Which of the following reaction occurs at cathode in H2 – O2 fuel cell?
(a) H+ + OH– → H2O
(b) O2 + 2H2O + 4e– → 4 OH–
(c) 2H2 + O2 > 2H2O
(d) H2 + 2OH– → 2H2O + 2e–
Answer
Answer: (b) O2 + 2H2O + 4e– → 4 OH–
Question 43.
Which of the following reaction occurs at anode during the recharging of lead storage battery?
(a) PbSO4 + 2H2O → PbO2 + SO\(_{ 4 }^{2-}\) +4H+ + 2e–
(b) Pb + S0\(_{ 4 }^{2-}\) → PbSO4 + 2e–
(c) PbSO4 + 2e– → Pb + S0\(_{ 4 }^{2-}\)
(d) PbO2 + 4H+ + SO\(_{ 4 }^{2-}\) + 2e– → PbSO4 + 2H2O
Answer
Answer: (a) PbSO4 + 2H2O → PbO2 + SO\(_{ 4 }^{2-}\) +4H+ + 2e–
Question 44.
In nickel-cadmium storage cell, the electrolyte is
(a) moist KOH
(b) dil H2SO4
(c) aqueous NH4Cl
(d) Ni(OH)3(aq)
Answer
Answer: (a) moist KOH
Assertion and Reason Type Questions
The questions given below consist of an assertion and a reason. Use the following key to choose the appropriate answer.
(a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
(b) Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanation of the Assertion (A).
(c) Assertion (A) is correct, Reason (R) is wrong statement.
(d) Assertion (A) is wrong, but Reason (R) is correct statement.
Question 45.
Assertion: Equivalent conductance of all electrolytes decreases with increasing concentration.
Reason: Lesser number of ions are available per gram equivalent at higher concentration.
Answer
Answer: (c) Assertion (A) is correct, Reason (R) is wrong statement.
Question 46.
Assertion: Iron is protected from corrosion by connecting magnesium metal with it. Reason: Iron acts as a cathode and magnesium as anode which gradually disappears.
Answer
Answer: (a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
Question 47.
Assertion: Chromium is used for coating iron.
Reason: Chromium is non-corroding metal which forms a protective layer on iron.
Answer
Answer: (a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
Question 48.
Assertion: Zinc can liberate H2 from aqueous solution of HCl.
Reason: Zinc has +ve reduction potential.
Answer
Answer: (c) Assertion (A) is correct, Reason (R) is wrong statement.
Question 49.
Assertion: Copper sulphate solution can be kept in a zinc vessel.
Reason: The position of zinc is higher than copper in the electrochemical series.
Answer
Answer: (d) Assertion (A) is wrong, but Reason (R) is correct statement.
Question 50.
Assertion: For CH3COOH, the molar
conductance of 0.1 M CH3COOH and equivalent conductance of 0.1 N CH3COOH is same.
Reason: These do not depend upon concentration.
Answer
Answer: (c) Assertion (A) is correct, Reason (R) is wrong statement.
Question 51.
Assertion: 0.1 M NH4OH at 25°C has more conductance than at 50°C.
Reason: Conductance of a weak electrolyte increases with increase in temperature.
Answer
Answer: (d) Assertion (A) is wrong, but Reason (R) is correct statement.
This NCERT MCQ Questions for Class 12 Chemistry Chapter 3 Electrochemistry with Answers Pdf free download has been put together to help students understand the CBSE Class 12 Chemistry Electrochemistry MCQs Multiple Choice Questions with Answers. Hope you found this helpful!