Do you need some help in preparing for your upcoming Class 11 Chemistry exams? We’ve compiled a list of MCQ on Thermodynamics Class 11 MCQs Questions with Answers to get you started with the subject. You can download NCERT MCQ Questions for Class 11 Chemistry Chapter 6 Thermodynamics with Answers Pdf free download, and learn how smart students prepare well ahead with MCQ Questions for Class 11 Chemistry with Answers.
Thermodynamics Class 11 MCQs Questions with Answers
Question 1.
Hess law is an application of
(a) 1st law of Thermodynamics
(b) 2nd law of Thermodynamics
(c) Entropy change
(d) ∆H = ∆U + P∆V.
Answer
Answer: (a) 1st law of Thermodynamics
Explanation:
Hesss law is an expression of the principle of conservation of energy, also expressed in the first law of thermodynamics, and the fact that the enthalpy of a chemical process is independent of the path taken from the initial to the final state (i.e. enthalpy is a state function).
Question 2.
5 mole of an ideal gas expand isothermally and irreversibly from a pressure of 10 atm to 1 atm against a constant external pressure of 1 atm. Wirr at 300 K is:
(a) -15.921 kJ
(b) -11.224 kJ
(c) -110.83 kJ
(d) None of these
Answer
Answer: (b) -11.224 kJ
Explanation:
P1V1 = RT η
10(V1) = 5(8.3)(300) 1
V1 = 150(0.0821)
P1V1 = P2V2
Therefore, 10(150 x0.0821) = 1 (V2)
Therefore V2 = 10(150 × 0.0821)
Therefore, -P Therefore V = -(1)(9)(150 × 0.0821) 101.3
= -11224 J = -11.224 KJ
Question 3.
At absolute zero the entropy of a perfect crystal is zero. This statement corresponds to which law of thermodynamics?
(a) Zeroth Law
(b) First Law
(c) Second Law
(d) Third Law
Answer
Answer: (d) Third Law
Explanation:
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has. Specifically, the entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy.
Question 4.
Which of the following has the highest entropy?
(a) Mercury
(b) Hydrogen
(c) Water
(d) Graphite
Answer
Answer: (b) Hydrogen
Explanation:
Gas has the highest entropy.
Question 5.
An ideal gas is taken through the cycle A → B → C → A as shown in figure. If the net heat supplied to the gas in cycle is 5 J, the work done by the gas in the process C → A.
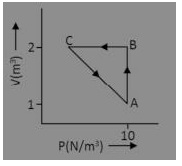
(a) -5 J
(b) -15 J
(c) -10 J
(d) -20 J
Answer
Answer: (a) -5 J
Explanation:
Work done by the gas is negative
∆ U in cyclic process is zero.
Therefore, w = -5J
Question 6.
One mole of which of the following has the highest entropy?
(a) Liquid Nitrogen
(b) Hydrogen Gas
(c) Mercury
(d) Diamond
Answer
Answer: (b) Hydrogen Gas
Explanation:
The measure of randomness of a substance is called entropy. Greater the randomness of molecules of a substance greater is the entropy. Here hydrogen gas has more entropy as it shows more randomness/disorderliness due to less molar mass than all the given substances and also in the gas phase.
Question 7.
An ideal gas is taken around the cycle ABCA as shown in P-V diagram The next work done by the gas during the cycle is equal to:
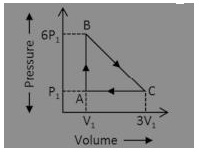
(a) 12P1V1
(b) 6P1V1
(c) 5P1V1
(d) P1V1
Answer
Answer: (c) 5P1V1
Explanation:
Work done = Area under P-V graph = (1/2) (5P1) (2V1) = 5P1 V1
Question 8.
Third law of thermodynamics provides a method to evaluate which property?
(a) Absolute Energy
(b) Absolute Enthalpy
(c) Absolute Entropy
(d) Absolute Free Energy
Answer
Answer: (c) Absolute Entropy
Explanation:
The Third Law of Thermodynamics is concerned with the limiting behavior of systems as the temperature approaches absolute zero. Most thermodynamics calculations use only entropy differences, so the zero point of the entropy scale is often not important. However, the Third Law tells us about the completeness as it describes the condition of zero entropy.
Question 9.
Which of the following is/are a reason that water is a desirable heat sink for use in calorimeters?
I) Waters heat specific capacity is very precisely known.
II) Water is readily available.
III) Water has an unusually large specific heat capacity.
(a) I only
(b) I and II
(c) I, II and III
(d) II only
Answer
Answer: (c) I, II and III
Explanation:
Water is a good heat sink for all of the reasons listed above. Moreover, its large heat capacity, liquid state and ready availability enable us to easily set up a calorimeter such that ∆T is large enough that it can be easily measured and small enough that phase transition temperatures are not reached.
Question 10.
In a chemical reaction the bond energy of reactants is more than the bond energy of the products. Therefore, the reaction is
(a) Exothermic
(b) Athermic
(c) Endothermic
(d) Endergonic
Answer
Answer: (c) Endothermic
Explanation:
If the products have a higher energy level than the reactants then the reaction is endothermic.
In an endothermic reaction, the reaction mixture absorbs heat from the surroundings. Therefore, the products will have a higher energy than the reactants and ΔH will be positive.
In an exothermic reaction, the reaction mixture releases heat to the surroundings. Therefore, the products will have a lower energy than the reactants and ΔH will be negative.
Question 11.
In a reversible process the system absorbs 600 kJ heat and performs 250 kJ work on the surroundings. What is the increase in the internal energy of the system?
(a) 850 kJ
(b) 600 kJ
(c) 350 kJ
(d) 250 kJ
Answer
Answer: (c) 350 kJ
Explanation:
∆E = q + w
= (600 – 250)
∆E = 350 J
Question 12.
Which of the following neutralisation reactions is most exothermic?
(a) HCl and NaOH
(b) HCN and NaOH
(c) HCl and NH4OH
(d) CH3COOH and NH4OH
Answer
Answer: (a) HCl and NaOH
Explanation:
Strong acid + Strong base → Most exothermic.
Question 13.
A student runs a reaction in a closed system. In the course of the reaction, 64.7 kJ of heat is released to the surroundings and 14.3 kJ of work is done on the system. What is the change in internal energy (∆U) of the reaction?
(a) -79.0 kJ
(b) 50.4 kJ
(c) 79.0 kJ
(d) -50.4 kJ
Answer
Answer: (d) -50.4 kJ
Explanation:
The change in internal energy is given as: ∆U = q + w. In this reaction, q is -64.7 kJ and w is 14.3 kJ. Therefore the correct answer is -50.4 kJ.
Question 14.
Identify the correct statement from the following in a chemical reaction.
(a) The entropy always increases
(b) The change in entropy along with suitable change in enthalpy decides the fate of a reaction
(c) The enthalpy always decreases
(d) Both the enthalpy and the entropy remain constant
Answer
Answer: (b) The change in entropy along with suitable change in enthalpy decides the fate of a reaction
Explanation:
ΔG = ΔH – TΔS
For a reaction to be spontaneous, ΔG should be negative. Therefore, resultant of ΔH and TΔS decide show the reaction will be carried.
Question 15.
2 mole of an ideal gas at 27° C expands isothermally and reversibly from a volume of 4 litres to 40 litre. The work done (in kJ) is:
(a) w = -28.72 kJ
(b) w = -11.488 kJ
(c) w = -5.736 kJ
(d) w = -4.988 kJ
Answer
Answer: (b) w = -11.488 kJ
Explanation:
w = -2.303 η RT log (V2/V1)
= -2.303(8 – 3) (300)(2) log (40/4)
= -114.8 100J
Question 16.
The latent heat of vapourization of ε liquid at 500 K and 1 atm pressure is 10.0 kcal/mol. What will be the change in internal energy (ΔU) of 3 moles of liquid at the same temperature
(a) 13.0 kcal/mol
(b) −13.0 kcal/mol
(c) 27.0 kcal
(d) −7.0 kcal/mol
Answer
Answer: (c) 27.0 kcal
Explanation:
3H2O(l) → 3H2O(g);
Δn = 3,
ΔE = ΔH − ΔnRT
= 30 − 3 × (2/1000) × 500
= 27 kcal
Question 17.
Calculate the heat required to make 6.4 Kg CaC2 from CaO(s) and C(s) from the reaction: CaO(s) + 3 C(s) → CaC2(s) + CO (g) given that ∆f H° (CaC2) = -14.2 kcal. ∆f H° (CO) = -26.4 kcal.
(a) 5624 kca
(b) 1.11 × 104 kcal
(c) 86.24 × 10³
(d) 1100 kcal
Answer
Answer: (b) 1.11 × 104 kcal
Explanation:
n = (Mass)/ (Molecular weight)
= (6.4 × 10³)/ (64)
= 100
For 1 mole of CaC2
∆ H = ∆Hf (CaC) + Hf (CO) – Hf (CaO)
= -14.2 – 26.4 + 151.6 = 111.1 kcal
For 100 moles, ∆H = 1.11 x 104 Kcal
Question 18.
Entropy of the universe is
(a) Continuously Increasing
(b) Continuously Decreasing
(c) Zero
(d) Constant
Answer
Answer: (a) Continuously Increasing
Explanation:
Energy always flows downhill, and this causes an increase of entropy. Entropy is the spreading out of energy, and energy tends to spread out as much as possible. The universe will have run down completely, and the entropy of the universe will be as high as it is ever going to get.
Question 19.
At absolute zero the entropy of a perfect crystal is zero. This statement corresponds to which law of thermodynamics?
(a) Zeroth Law
(b) First Law
(c) Second Law
(d) Third Law
Answer
Answer: (d) Third Law
Explanation:
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has. Specifically, the entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy.
Question 20.
The bond energy (in kcal mol-1) of a C-C single bond is approximately
(a) 1
(b) 10
(c) 83-85
(d) 1000
Answer
Answer: (c) 83-85
Explanation:
C–C bond 83–85 kcal/mol
It is the energy required to break the bond .It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K (absolute zero)
We hope you found this CBSE Class 11 Chemistry Thermodynamics MCQs Multiple Choice Questions with Answers helpful. If you have any questions about NCERT MCQ Questions for Class 11 Chemistry Chapter 6 Thermodynamics with Answers Pdf free download, please share them in the comment box below and we will get back to you at the earliest possible time.