Do you need some help in preparing for your upcoming Class 11 Chemistry exams? We’ve compiled a list of MCQ on The p-Block Elements Class 11 MCQs Questions with Answers to get you started with the subject. You can download NCERT MCQ Questions for Class 11 Chemistry Chapter 11 The p-Block Elements with Answers Pdf free download, and learn how smart students prepare well ahead with MCQ Questions for Class 11 Chemistry with Answers.
The p-Block Elements Class 11 MCQs Questions with Answers
Solving the The p-Block Elements Multiple Choice Questions of Class 11 Chemistry Chapter 11 MCQ can be of help to learn the concepts.
P-block elements:
The s-block and p-block elements are so called because their valence electrons are in an s orbital or p orbital respectively. They are also called Typical Elements to distinguish them from the transition and inner transition series. Consequently there are six groups of p–block elements in the periodic table numbering from 13 to 18. Boron, carbon, nitrogen, oxygen, fluorine and helium head the groups.
Question 1.
Consider the following statement about Ozone I. O3 is formed by the interaction of fluorine. II. It turns tetramethyl base paper as violet. III. It turns benzidine paper as brown. The correct set of true statement is
(a) I and II
(b) I, II and III
(c) I and III
(d) II and III
Answer
Answer: (b) I, II and III
Explanation:
Ozone is formed by the interaction of fluorine. It turns tetramethyl base paper and benzidine paper as violet and brown respectively.
Hence, the correction option is (2).
Question 2.
In the compound of type ECl3, where E = B, P, As, or Bi, the angle Cl – E – Cl for different E are ion the order:
(a) B = P = As = Bi
(b) B > P > As > Bi
(c) B < P = As = Bi
(d) B < P < As < Bi
Answer
Answer: (b) B > P > As > Bi
Explanation:
BCl3 is trigonal planar in structure and bond angles are 120° each. PCl3, AsCl3, and BiCl3 are pyramidal in shape with sp³-hybridization.
In all of them, the bond angles are less than the normal tetrahedral angle of 109.28, and also these bond angles decrease down the group.
Therefore, the correct order of bond angles is as follows:
B > P > As > Bi
Question 3.
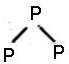
In white phosphorous(P4) molecule, which one is not correct:
(a) 6P-P single bonds are present
(b) 4P-P single bonds are present
(c) 4 lone pair of electrons is present
(d) P-P-P bond angle is 60°
Answer
Answer: (a) 6P-P single bonds are present
Explanation:
It has total four lone pairs of electrons situated at each P – atom.
It has six P_P single bond
Question 4.
All the elements of oxygen family are
(a) Non metals
(b) Metalloids
(c) Radioactive
(d) Polymorphic
Answer
Answer: (d) Polymorphic
Explanation:
Group 16 elements are called polymorphic elements because all elements show allotropy except Te.
Question 5.
Which of the following will not produce hydrogen gas ?
(a) Reaction between Fe and dil. HCl
(b) Reaction between Zn and NaOH
(c) Reaction between Zn and conc. H2SO4
(d) Electrolysis of NaCl in Nelsons cell
Answer
Answer: (c) Reaction between Zn and conc. H2SO4
Explanation:
Concentrated sulphuric acid reacts with Zn to give SO2 and not H2
Question 6.
Amorphous form of Silica is
(a) Tridymite
(b) Kieselguhr
(c) Cristobalite
(d) Quartz
Answer
Answer: (c) Cristobalite
Explanation:
Silicon Dioxide/ Silica/ Quartz –
Covalent, three dimensional solid network in which each silicon is covalently bond to four oxygen atoms (sp³ hybridisation) forming a tetrahedral structure.
Function of quartz –
As piezoelectric material in clocks, radio, television broadcasting and mobile communication.
Quartz, tridymite, cristobalite is crystalline form, and kieselguhr is an amorphous form of silica.
Question 7.
Graphite is a soft solid lubricant extremely diffcult to melt. The reason for this anomalous behaviour is that graphite.
(a) Has carbon atoms arranged in large plates of rings of strongly bound carbon atoms with weak interplate bonds
(b) Is a non – crystalline substance
(c) Is an allotropic from of carbon
(d) Has molecules of variable molecular masses like polymers.
Answer
Answer: (a) Has carbon atoms arranged in large plates of rings of strongly bound carbon atoms with weak interplate bonds
Explanation:
C-atoms oi graphite form covalently bonded plates (layers) These layers are held together by weak forces of attraction. i.e., one layer can slide over other to cause lubricacy. lt cannot be melted easily as a large number of atoms being bonded strongly in the layer to form big entity.
Question 8.
Borax is used as a cleansing agent because on dissolving in water, it gives
(a) Alkaline solution
(b) Acidic solution
(c) Bleaching solution
(d) Amphoteric solution.
Answer
Answer: (a) Alkaline solution
Explanation:
Borax dissolves in water to give an alkaline solution.
Na2B4O7 + 7H2O ⇔ 2NaOH + 4H3BO3.
Question 9.
Among the C-X bond (where, X = Cl, Br, I) the correct decreasing order of bond energy is
(a) C−I > C−Cl > C−Br
(b) C−I > C−Br > C−Cl
(c) C−Cl > C−Br > C−I
(d) C−Br > C−Cl > C−I
Answer
Answer: (c) C−Cl > C−Br > C−I
Explanation:
Among the C-X bond (where, X = Cl, Br, I), the correct decreasing order of bond energy is
C−Cl > C−Br > C−l
Question 10.
On heating boron with caustic potash, the pair of products formed are
(a) Potassium Borate + Dihydrogen
(b) Potassium Borate + Water
(c) Potassium Borate + H2
(d) Borax + Dihydrogen.
Answer
Answer: (a) Potassium Borate + Dihydrogen
Explanation:
2B + 2KOH + 2H2O → 2KBO2 + 3H2
Boron react with potassium hydroxide and water to produce potassium metaborate and hydrogen.
Question 11.
Which of the following statements regarding ozone is not correct?
(a) The oxygen-oxygen bond length in ozone is identical with that of molecular oxygen
(b) The ozone is response hybrid of two structures
(c) The ozone molecule is angular in shape
(d) Ozone is used as a germicide and disinfectant for the purification of air.
Answer
Answer: (a) The oxygen-oxygen bond length in ozone is identical with that of molecular oxygen
Explanation:
The oxygen–oxygen bond length in ozone is identical with that of molecular oxygen
Question 12.
There is no S-S bond in
(a) S2O2-4
(b) S2O2-5
(c) S2O2-3
(d) S2O2-7
Answer
Answer: (d) S2O2-7
Solution :
There is no S-S bond in S2O2-7

Question 13.
Which is strongest Lewis acid?
(a) BF3
(b) BCl3
(c) BBr3
(d) BI3
Answer
Answer: (a) BF3
Explanation:
Larger the size of halogen atom less is the back donation of electrons into empty 2p orbital of B.
Question 14.
Fertilizer having the highest nitrogen percentage is:
(a) Calcium cyanamide
(b) Urea
(c) Ammonium nitrate
(d) Ammonium sulphate
Answer
Answer: (b) Urea
Explanation:
Every compound has 2N atoms (i.e., same mass of N), thus compound with the lowest molecular mass (i.e., urea) will have the highest N percentage.
Question 15.
In general, the Boron Trihaides act as
(a) Strong reducing agent
(b) Lewis Acids
(c) Lewis Bases
(d) Dehydrating Agents
Answer
Answer: (b) Lewis Acids
Explanation:
The boron atom in trihaldies has only six electrons in the valence shell and hence can accept a pair of electrons in the vacant p-orbital to complete its octet. As a result, boron trihaldies act as a Lewis acids.
Question 16.
Which of the following is not a mineral of boron?
(a) Colemanite
(b) Kernite
(c) Boric Anhydride
(d) Borax
Answer
Answer: (c) Boric Anhydride
Explanation:
The most important boron minerals in commercial terms are; Tincal, Colemanite, Kernite, Ulexite, Pandermite, Boracite, Szaybelite and Hydroboracite. The main boron minerals transformed by Eti Maden, the World Boron Leader, into high value added products in international quality standards are; Tincal, Colemanite and Ulexite.
Question 17.
Which phosphorus is used as a rat poison?
(a) White
(b) Violet
(c) Red
(d) Black
Answer
Answer: (a) White
Explanation:
White phosphorous is least stable and most toxic of all allotropes. Upon coming on contact with air it is toxic and causes severe liver damage on digestion so it is used as rat poison.
Question 18.
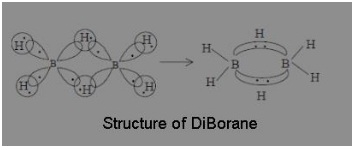
The structure of diBorane contains
(a) Four 2c – 2e bonds and two 3c – 2e bonds
(b) Two 2c – 2e bonds and two 3c – 2e bonds
(c) Two 2c – 2e bonds and two 3c – 3e bonds
(d) Four 2c – 2e bonds and four 3c – 2e bonds
Answer
Answer: (a) Four 2c – 2e bonds and two 3c – 2e bonds
Explanation:
According to molecular orbital theory, each of the two boron atoms is in sp³ hybrid state. Of the four hybrid orbitals, three have one electron each while the fourth is empty. Two of the four orbitals of each of the boron atom overlap with two terminal hydrogen atoms forming two normal B – H σ-bonds. One of the remaining hybrid orbital (either filled or empty) of one of the boron atoms, 1s orbital of hydrogen atoms (bridge atom) and one of hybrid orbitals of the other boron atom overlap to form a delocalised orbital covering the three nuclei with a pair of electrons. Such a bond is known as three centre two electron (3c – 2e) bonds
Question 19.
Nitrogen (I) oxide is produced by:
(a) Thermal decomposition of ammonium nitrate
(b) Disproportionation of N2O4
(c) Thermal decomposition of ammonium nitrite
(d) None of the above
Answer
Answer: (c) Thermal decomposition of ammonium nitrite
Explanation:
Nitrous Oxide (N2O) can be produced by thermal decomposition of ammonium nitrate:
NH4NO3(s) → N2O (g) + 2H2O(l)
Question 20.
Red phosphorus is chemically less reactive because
(a) It does not contain P – P bonds
(b) It dos not contain tetrahedral P4 molecules
(c) It does not catch fire in air even upto 400°C
(d) It has a polymeric structure
Answer
Answer: (d) It has a polymeric structure
Explanation:
Red phosphorus is less reactive because of its gaint polymeric structure.
We hope you found this CBSE Class 11 Chemistry The p-Block Elements MCQs Multiple Choice Questions with Answers helpful. If you have any questions about NCERT MCQ Questions for Class 11 Chemistry Chapter 11 The p-Block Elements with Answers Pdf free download, please share them in the comment box below and we will get back to you at the earliest possible time.